class 7 heat notes
Class 7 – Heat
Questions covered are
1. What is heat, and how is it different from temperature?
2. Describe the construction and working of a clinical thermometer.
3. Explain the differences between a clinical thermometer and a laboratory thermometer.
4. What are conductors and insulators of heat? Provide examples of each.
5. Describe the three modes of heat transfer with examples.
6. Why do we prefer to wear light-colored clothes in summer and dark-colored clothes in winter?
7. Why are stainless steel cooking utensils often provided with copper bottoms?
8. Explain why it is advisable to paint the outer walls of houses white in hot climates.
9. Discuss the importance of the kink (constriction) in a clinical thermometer.
10. Why does the temperature of boiling water remain constant even when heated further?
11. Why do we feel hot air rising above a flame?
12. Why do we use woolen clothes in winter?
13. Why does a metal spoon feel colder than a wooden spoon at room temperature?
14. Why are handles of cooking utensils made of wood or plastic?
15. Why do houses in cold regions have chimneys?
16. Why do the bottoms of cooking utensils turn black after prolonged use?
17. Why is land warmer than water during the day and cooler at night?
18. What is the sea breeze and land breeze?
19. Why do we use thermos flasks to keep liquids hot or cold?
20. Why do black surfaces absorb more heat than white surfaces?
***************************************************************************
1. What is heat, and how is it different from temperature?
| Feature | Heat | Temperature |
| Definition | A form of energy that transfers between objects due to a temperature difference. | A measure of the hotness or coldness of an object. |
| Symbol | Represented by Q. | Represented by T. |
| Unit of Measurement | Measured in joules (J) or calories (cal). | Measured in degrees Celsius (°C), Fahrenheit (°F), or Kelvin (K). |
| Instrument Used | Measured using a calorimeter. | Measured using a thermometer. |
| Depends On | Depends on the mass of the object, specific heat capacity, and temperature change. | Depends on the average kinetic energy of particles in a substance. |
| Can It Flow? | Yes, heat flows from a hotter object to a cooler object. | No, temperature is just an indication of heat energy but does not flow. |
| Example | The heat from the Sun warms the Earth. | Boiling water has a temperature of 100°C. |
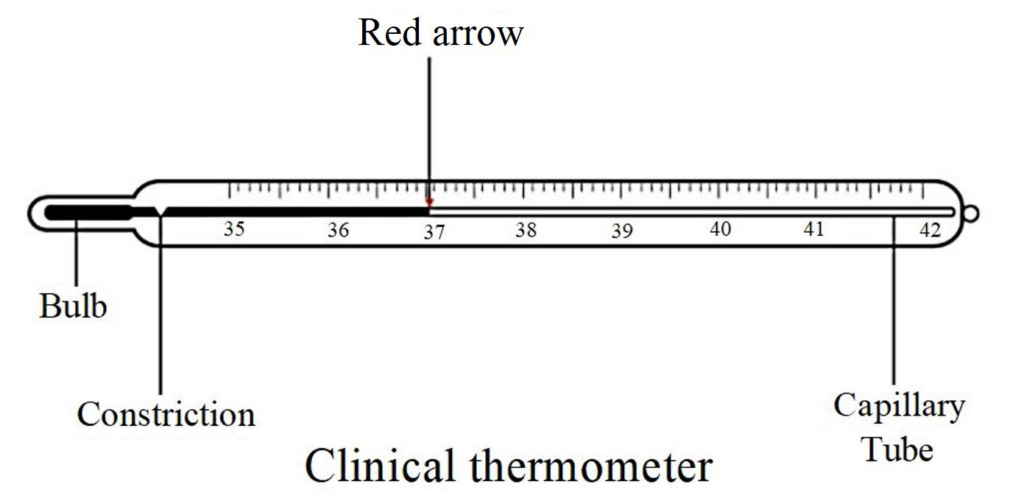
2. Describe the construction and working of a clinical thermometer.
Construction:
- A narrow, uniform glass tube with a bulb at one end containing mercury.
- A constriction near the bulb prevents mercury from falling back, allowing the reading to be retained.
- The scale ranges from 35°C to 42°C, suitable for measuring human body temperature.
Working:
- When placed under the tongue or armpit, body heat causes the mercury to expand and rise in the tube.
- The maximum expansion indicates the body temperature, which can be read after removing the thermometer.
- The constriction ensures the mercury column stays in place until reset by shaking.
3. Explain the differences between a clinical thermometer and a laboratory thermometer.

| Feature | Clinical Thermometer | Laboratory Thermometer |
| Purpose | Measures human body temperature | Measures temperature in experiments |
| Temperature Range | 35°C to 42°C | -10°C to 110°C |
| Presence of Kink (Constriction) | Yes, to hold mercury in place for accurate reading | No, mercury moves freely |
| Use | Used in homes, hospitals, and clinics | Used in laboratories for scientific research |
| Reading Time | Can be read after removal due to the kink | Must be read while in contact with the substance |
| Liquid Inside | Mercury or alcohol | Mercury or alcohol |
| Glass Tube Thickness | Thicker for durability and safety | Usually thinner than clinical thermometers |
4. What are conductors and insulators of heat? Provide examples of each.
| Property | Conductors of Heat | Insulators of Heat |
| Definition | Materials that allow heat to pass through them easily. | Materials that do not allow heat to pass through them easily. |
| Thermal Conductivity | High thermal conductivity. | Low thermal conductivity. |
| Examples (Metals & Non-Metals) | Metals like copper, aluminum, iron, and silver. | Non-metals like wood, rubber, plastic, and glass. |
| Examples (Everyday Use) | Cooking utensils, metal rods, and kettles. | Woolen clothes, thermos flasks, and foam. |
| Heat Transfer | Heat flows quickly through conductors. | Heat transfer is slow through insulators. |
5. Describe the three modes of heat transfer with examples.
| Mode of Heat Transfer | Description | Examples |
| Conduction | Heat transfer through direct contact between particles in a solid. Heat moves from the hotter part to the cooler part. | – A metal spoon getting hot when placed in a hot cup of tea. – Heating one end of an iron rod makes the other end hot. – Cooking pans transferring heat from the stove to food. |
| Convection | Heat transfer in liquids and gases due to the movement of heated particles. Warm fluid rises, and cooler fluid sinks, creating a circulation. | – Boiling water in a pot (hot water rises, cold water sinks). – Land and sea breezes (air movement due to temperature differences). – Hot air balloons rising as warm air expands. |
| Radiation | Heat transfer through electromagnetic waves without needing a medium (can happen in a vacuum). | – The Sun’s heat reaching the Earth. – Feeling warmth from a campfire without touching it. – A heated iron rod glowing red and radiating heat. |
6. Why do we prefer to wear light-colored clothes in summer and dark-colored clothes in winter?
Light-Colored Clothes in Summer:
- Reflect most of the sunlight, absorbing less heat.
- Help keep the body cooler in hot weather.
Dark-Colored Clothes in Winter:
- Absorb more sunlight, retaining more heat.
- Help keep the body warmer in cold weather.
7. Why are stainless steel cooking utensils often provided with copper bottoms?
- Copper is an excellent conductor of heat, allowing for uniform heating.
- Stainless steel is durable but not as good a conductor as copper.
- Combining both materials ensures even cooking and longevity of the utensil.
8. Explain why it is advisable to paint the outer walls of houses white in hot climates.
- White surfaces reflect a significant portion of sunlight.
- This reflection reduces heat absorption, keeping the interior cooler.
- Helps in maintaining a comfortable indoor temperature in hot climates.
9. Discuss the importance of the kink (constriction) in a clinical thermometer.
Importance:
- The constriction prevents mercury from falling back into the bulb after removing the thermometer from the body.
- Allows the user to read the temperature accurately without haste.
- Ensures that the measured temperature remains displayed until manually reset.
10. Why does the temperature of boiling water remain constant even when heated further?
- At boiling point, water undergoes a phase change from liquid to gas.
- The added heat energy is used for this phase transition, known as latent heat of vaporization.
- As a result, the temperature remains constant until all the water has vaporized.
11. Why do we feel hot air rising above a flame?
- When air is heated, it expands and becomes less dense.
- The lighter, hot air rises while cooler, denser air moves in to take its place.
- This process is called convection, and it explains why we feel hot air rising above a flame.
12. Why do we use woolen clothes in winter?
- Wool is a poor conductor of heat, preventing heat loss from the body.
- Wool fibers trap air, which acts as an insulator, keeping us warm.
- Wearing woolen clothes reduces heat loss and provides insulation against the cold.
13. Why does a metal spoon feel colder than a wooden spoon at room temperature?
- Metal is a good conductor of heat, so it quickly absorbs heat from your hand, making it feel cold.
- Wood is a poor conductor (insulator), so it does not draw heat away as quickly, making it feel warmer.
- Even though both objects are at the same room temperature, the way they conduct heat affects how we perceive their temperature.
14. Why are handles of cooking utensils made of wood or plastic?
- Wood and plastic are poor conductors of heat, preventing burns when holding the utensil.
- They provide a comfortable grip without becoming too hot.
- These materials also offer electrical insulation, ensuring safety in kitchen appliances.
15. Why do houses in cold regions have chimneys?
- Hot air rises due to convection, carrying smoke and unwanted gases upward.
- Chimneys provide an escape route for hot air, preventing indoor air pollution.
- This design maintains proper ventilation and helps in keeping the house warm.
16. Why do the bottoms of cooking utensils turn black after prolonged use?
- During cooking, incomplete combustion of fuel produces soot (carbon particles).
- These carbon deposits accumulate on the bottom of the utensil, turning it black.
- Using clean-burning fuel and proper ventilation can reduce soot formation.
17. Why is land warmer than water during the day and cooler at night?
- Land heats up faster than water during the day due to its lower heat capacity.
- At night, land cools down faster than water because it also loses heat more quickly.
- This difference in heating and cooling leads to land and sea breezes in coastal areas.
18. What is the difference between sea breeze and land breeze?
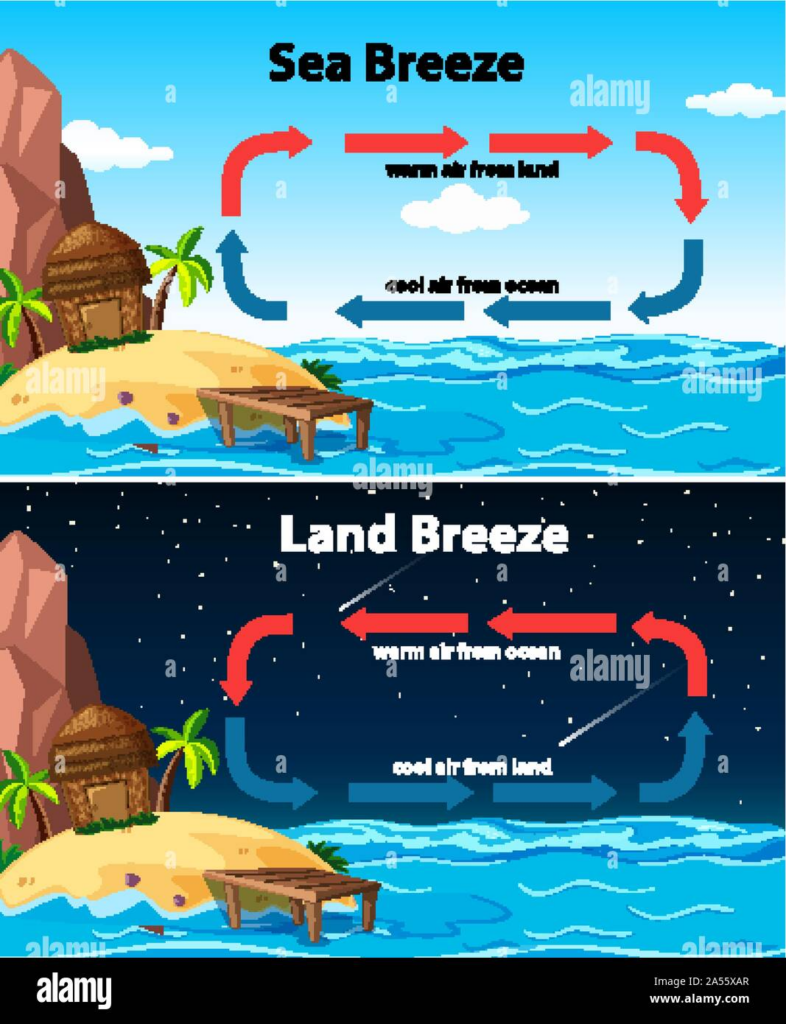
| Feature | Sea Breeze | Land Breeze |
| Definition | The movement of cool air from the sea to the land during the day. | The movement of cool air from the land to the sea during the night. |
| Time of Occurrence | Happens during the daytime. | Happens during the nighttime. |
| Reason | Land heats up faster than water, so warm air over the land rises, and cool air from the sea moves in to replace it. | Land cools down faster than water, so warm air over the sea rises, and cool air from the land moves in to replace it. |
| Direction of Wind | From sea to land. | From land to sea. |
| Effect on Temperature | Cools down coastal areas during the day. | Warms up coastal areas during the night. |
| Example | A cool breeze is felt near the shore during the afternoon. | A gentle breeze blows from land toward the sea at night. |
19. Why do we use thermos flasks to keep liquids hot or cold?
- A thermos flask has a vacuum layer between its inner and outer walls, reducing heat transfer by conduction and convection.
- The inner surface is silvered, minimizing heat transfer by radiation.
- This insulation helps maintain the temperature of the liquid inside for a long time.
20. Why do black surfaces absorb more heat than white surfaces?
- Black surfaces absorb more heat because they absorb all wavelengths of light and convert them into heat.
- White surfaces reflect most of the light and absorb very little heat.
- This is why black clothes feel hotter in the sun, while white clothes remain cooler.
Useful links: