class 7 acid, bases and salt pdf
Class 7 Acid, Bases and Salt
Questions Covered:
1.What are Acids? Give Examples
2.What are Bases? Give Examples.
3.What are Indicators? Name Three Natural Indicators.
4.Difference Between Acids and Bases?
5.What is Neutralization? Explain with an Example.
6.Where do we see neutralization in daily life?
7.What is Acid Rain? How Does It Affect the Environment?
In-Text Questions with Answers
Exercise Questions & Answers – Acids, Bases, and Salts
***************************************************************************
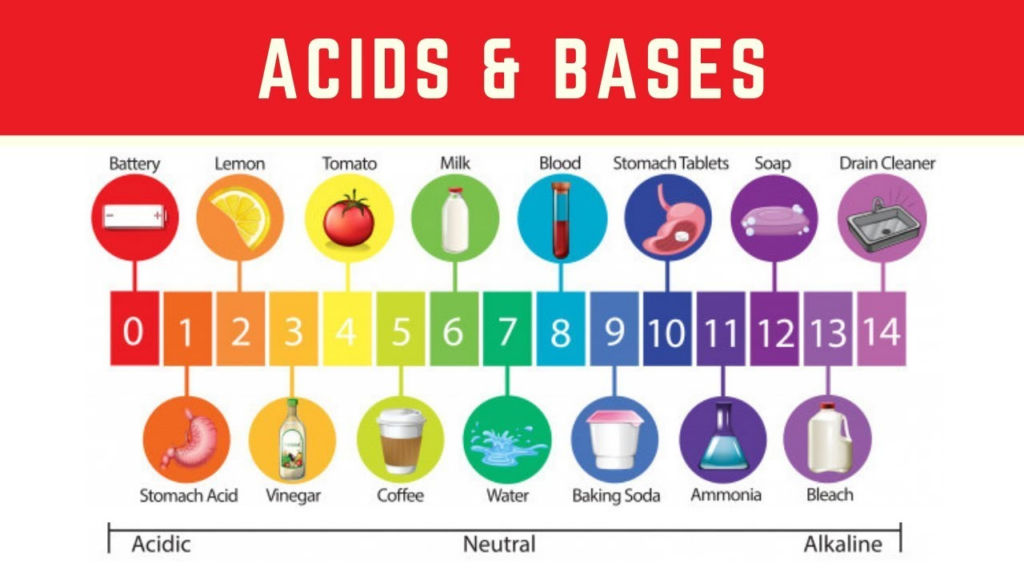
1.What are Acids? Give Examples
Acids:
- Acids are chemical substances that have a sour taste and can turn blue litmus red.
- They contain hydrogen ions (H⁺) when dissolved in water, which gives them their acidic properties.
- The word “acid” comes from the Latin word “acere,” meaning sour.
- Acids can be naturally occurring (found in plants and animals) or synthetically made in laboratories.
Properties of Acids:
- Sour in taste (e.g., lemon juice, vinegar).
- Corrosive in nature (strong acids can damage metals and skin).
- Turn blue litmus red (Litmus is an indicator used to detect acids and bases).
- React with metals to produce hydrogen gas.
- React with bases to undergo neutralization, forming salt and water.
- Conduct electricity when dissolved in water (because they produce H⁺ ions).
Types of Acids:
Acids can be classified into two types based on their sources:
- Natural Acids (Organic Acids): Found in plants and animals.
- Synthetic Acids (Mineral Acids): Made in laboratories and used in industries.
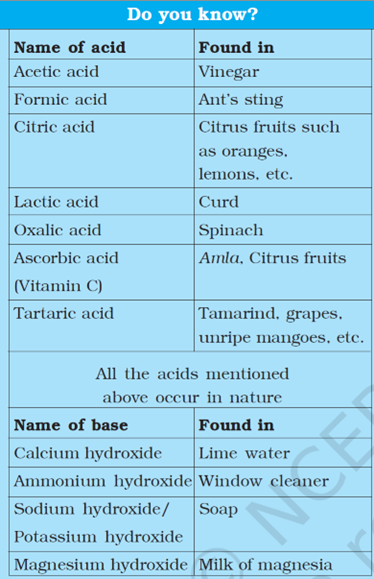
Examples of Acids in Everyday Life:
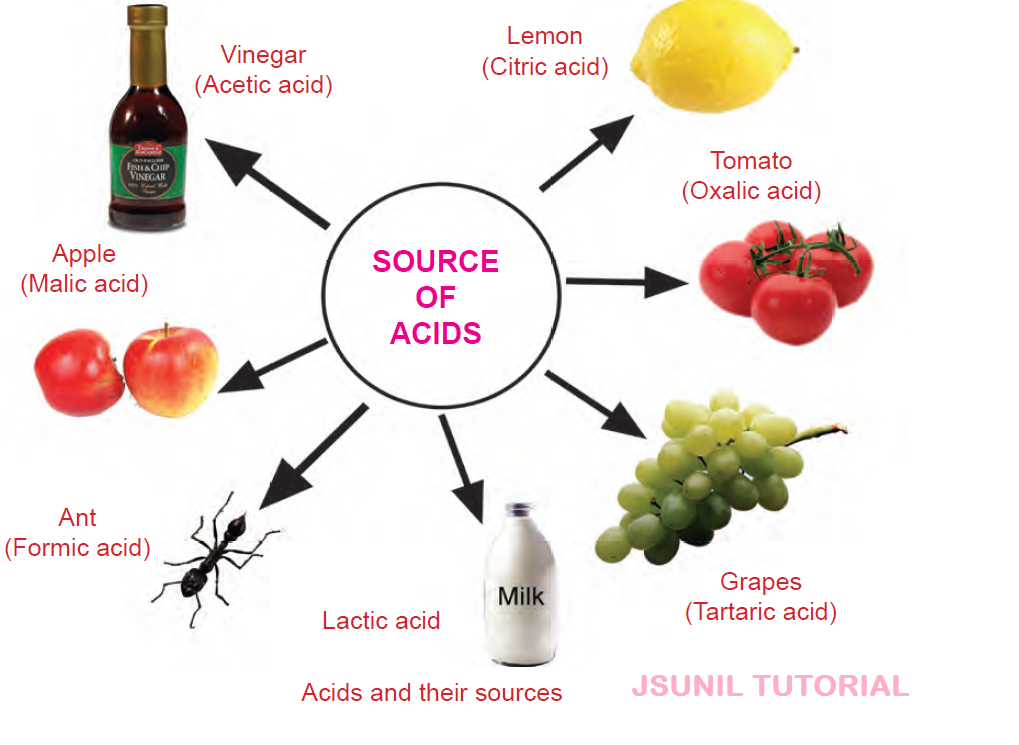
| Type of Acid | Commonly Found In |
| Acetic Acid (CH₃COOH) | Vinegar |
| Citric Acid | Citrus fruits (Lemon, Orange) |
| Tartaric Acid | Tamarind, Grapes, Unripe Mangoes |
| Lactic Acid | Curd, Sour Milk |
| Oxalic Acid | Spinach, Tomato |
| Formic Acid | Ant’s Sting, Bee Sting |
| Ascorbic Acid (Vitamin C) | Amla, Citrus Fruits |
Examples of Synthetic Acids:
| Name of Acid | Uses |
| Hydrochloric Acid (HCl) | Used in the digestive system (stomach acid) and cleaning products. |
| Sulfuric Acid (H₂SO₄) | Used in car batteries and manufacturing fertilizers. |
| Nitric Acid (HNO₃) | Used in fertilizers, explosives, and chemical industries. |
| Carbonic Acid (H₂CO₃) | Found in aerated (soft) drinks. |
| Phosphoric Acid (H₃PO₄) | Used in food preservatives and soft drinks. |
Uses of Acids in Daily Life:
- Vinegar (Acetic acid): Used in cooking and food preservation.
- Citric acid: Used in fruit juices and medicines (Vitamin C).
- Lactic acid: Used in curd, cheese, and buttermilk production.
- Sulfuric acid: Used in car batteries and fertilizers.
- Hydrochloric acid: Found in the stomach to aid digestion.
2.What are Bases? Give Examples.
Bases:
- Bases are chemical substances that have a bitter taste and feel soapy or slippery when touched.
- They turn red litmus blue and react with acids to form salt and water in a reaction called neutralization.
- Bases contain hydroxide ions (OH⁻) when dissolved in water, which gives them their basic properties.
Properties of Bases:
- Bitter in taste (e.g., soap, baking soda).
- Slippery or soapy to touch (e.g., soap, detergent).
- Turn red litmus paper blue.
- React with acids to undergo neutralization, forming salt and water.
- Some bases are soluble in water, and these are called alkalis.
- Strong bases can be corrosive (e.g., sodium hydroxide).
Types of Bases:
Bases can be classified into two types based on their solubility in water:
- Alkalis (Water-Soluble Bases): Bases that dissolve in water.
- Examples: Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Calcium hydroxide (Ca(OH)₂).
- Insoluble Bases: Bases that do not dissolve in water.
- Examples: Copper hydroxide (Cu(OH)₂), Zinc hydroxide (Zn(OH)₂), Iron hydroxide (Fe(OH)₃).
Examples of Bases in Everyday Life:
| Name of Base | Commonly Found In |
| Sodium hydroxide (NaOH) | Soap, detergents |
| Potassium hydroxide (KOH) | Shaving cream, liquid soap |
| Calcium hydroxide (Ca(OH)₂) | Lime water, whitewashing |
| Magnesium hydroxide (Mg(OH)₂) | Milk of magnesia (used for indigestion) |
| Ammonium hydroxide (NH₄OH) | Window cleaners |
| Baking soda (Sodium bicarbonate – NaHCO₃) | Baking, antacids, fire extinguishers |
Uses of Bases in Daily Life:
- Soaps and Detergents: Contain sodium hydroxide (NaOH) and potassium hydroxide (KOH), which help in cleaning.
- Antacids: Magnesium hydroxide (Milk of Magnesia) neutralizes excess stomach acid.
- Whitewashing Walls: Calcium hydroxide (lime water) is used in whitewashing.
- Baking Soda (NaHCO₃): Used in baking and fire extinguishers.
- Household Cleaning Products: Ammonium hydroxide (NH₄OH) is used in window cleaners.
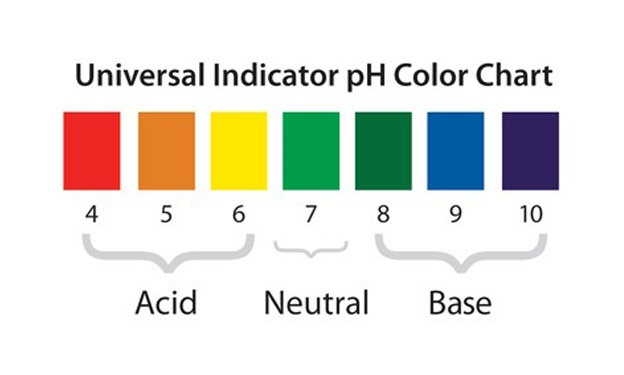
3.What are Indicators? Name Three Natural Indicators.
Indicators:
- Indicators are substances that change colour when added to an acid or a base.
- They help us identify whether a substance is acidic, basic, or neutral.
- Indicators can be natural (obtained from plants) or synthetic (made in laboratories).
Types of Indicators:
- Natural Indicators: Obtained from natural sources like plants and flowers.
- Example: Litmus, Turmeric, China Rose.
- Synthetic Indicators: Made in laboratories and used in chemical testing.
- Example: Phenolphthalein, Methyl Orange.
- Olfactory Indicators: Change smell in the presence of acids or bases.
- Example: Vanilla extract, Onion extract.
Three Natural Indicators and Their Uses:
| Indicator | Source | Effect in Acid | Effect in Base |
| Litmus | Lichens | Turns red | Turns blue |
| Turmeric | Turmeric plant | No change (remains yellow) | Turns red |
| China Rose (Hibiscus) | China rose petals | Turns dark pink (magenta) | Turns green |
4.Difference Between Acids and Bases?
| Property | Acids | Bases |
| Definition | Acids are substances that produce hydrogen ions (H⁺) when dissolved in water. | Bases are substances that produce hydroxide ions (OH⁻) when dissolved in water. |
| Taste | Sour (e.g., lemon juice, vinegar). | Bitter (e.g., soap, baking soda). |
| Touch | Not slippery, but strong acids can burn the skin. | Slippery or soapy to touch. |
| Effect on Litmus Paper | Turns blue litmus red. | Turns red litmus blue. |
| Effect on Turmeric Indicator | No change (remains yellow). | Turns red. |
| Effect on China Rose Indicator | Turns dark pink (magenta). | Turns green. |
| Reaction with Metals | Reacts with metals like zinc and magnesium to produce hydrogen gas. | Does not react with metals. |
| Reaction with Bases | Neutralizes bases to form salt and water. | Neutralizes acids to form salt and water. |
| Examples in Daily Life | Lemon juice, vinegar, curd, tamarind, citrus fruits. | Soap, baking soda, toothpaste, milk of magnesia. |
5.What is Neutralization? Explain with an Example.
- Neutralization is a chemical reaction in which an acid reacts with a base to form salt and water.
- This reaction removes the acidic and basic properties of the substances involved.
- Heat is also produced during neutralization, making it an exothermic reaction.
Neutralization Reaction Equation:
Acid+Base→Salt+Water+Heat
Hydrochloric acid (HCl)+Sodium hydroxide (NaOH)→Sodium chloride (NaCl)+Water (H₂O
6.Where do we see neutralization in daily life?
Daily Life Examples of Neutralization:
| Situation | Explanation |
| Indigestion | The stomach produces hydrochloric acid (HCl) to digest food. Too much acid causes indigestion. Antacids (e.g., milk of magnesia – Mg(OH)₂) neutralize the excess acid. |
| Ant Bite | Ants inject formic acid into the skin. Applying baking soda (NaHCO₃) or calamine lotion (ZnCO₃) neutralizes the acid and reduces irritation. |
| Soil Treatment | Excess use of fertilizers makes the soil acidic. Adding lime (CaO) or slaked lime (Ca(OH)₂) neutralizes the soil, making it suitable for crops. |
| Factory Waste | Many factories release acidic waste into rivers, which can kill fish and plants. Adding a base neutralizes the acids before disposal. |
7.What is Acid Rain? How Does It Affect the Environment?
Acid Rain:
- Acid rain is rainwater that contains harmful acids formed when pollutants like sulfur dioxide (SO₂) and nitrogen oxides (NO₂) mix with water in the atmosphere.
- It is caused by pollution from factories, vehicles, and burning fossil fuels.
- The acids formed in acid rain include sulfuric acid (H₂SO₄), nitric acid (HNO₃), and carbonic acid (H₂CO₃).
How Is Acid Rain Formed?
- Factories, power plants, and vehicles release sulfur dioxide (SO₂) and nitrogen oxides (NO₂) into the air.
- These gases react with water vapour in the atmosphere to form acids.
- The acids mix with rainwater and fall to the Earth as acid rain.
Chemical Reactions in Acid Rain Formation:
Sulfur dioxide (SO₂)+Oxygen (O₂)+Water (H₂O)→Sulfuric Acid (H₂SO₄)
Sulfur dioxide (SO₂)+Oxygen (O₂)+Water (H₂O)→Sulfuric Acid (H₂SO₄)
Nitrogen oxides (NO₂)+Water (H₂O)→Nitric Acid (HNO₃)
Effects of Acid Rain on the Environment:
| Affected Area | Effects of Acid Rain |
| Plants & Trees | – Damages leaves and slows down photosynthesis. – Makes the soil too acidic, affecting plant growth. |
| Water Bodies (Rivers, Lakes, Ponds) | – Lowers the pH of water, making it too acidic for fish and aquatic life. – Can kill fish and other aquatic animals. |
| Soil | – Washes away important minerals needed for plant growth. – Reduces soil fertility. |
| Buildings & Monuments | – Reacts with limestone and marble, causing them to weaken and decay. – Famous monuments like the Taj Mahal are damaged due to acid rain. |
| Human Health | – Increases the risk of breathing problems like asthma and lung diseases. – Pollutes drinking water. |
How Can We Reduce Acid Rain?
- Use clean energy sources like solar and wind power instead of burning fossil fuels.
- Reduce pollution by using catalytic converters in vehicles.
- Use fuels with low sulphur content.
- Plant more trees to absorb carbon dioxide and reduce air pollution.
- Treat industrial waste gases before releasing them into the air.
In-Text Questions with Answers
Q1: Do all substances have the same taste?
- No, different substances have different tastes.
- Some substances are sour (e.g., lemon juice, vinegar), some are bitter (e.g., baking soda, soap), some are salty (e.g., common salt), and some are sweet (e.g., sugar).
Q2: Why do some substances taste sour?
- Substances that taste sour contain acids.
- These substances are acidic in nature.
- Example: Curd, lemon juice, vinegar, orange juice, tamarind contain acids.
Q3: What is the taste of baking soda? Is it acidic?
- Baking soda does not taste sour; instead, it is bitter in taste.
- It is not acidic; it is a base.
- Bases are substances that are bitter and feel soapy when touched.
Q4: How can we test whether a substance is acidic or basic?
- We can use indicators to test if a substance is acidic or basic.
- Indicators change their color in the presence of acids or bases.
- Example: Litmus, Turmeric, and China Rose indicators.
Q5: What is an indicator? Name some natural indicators.
- Indicators are substances that change color when added to an acid or a base.
- Natural indicators:
- Litmus (from lichens)
- Turmeric
- China Rose petals
Q6: What happens when you put a drop of soap solution on turmeric paper?
- Turmeric turns red when soap solution is added.
- Explanation:
- Turmeric is a natural indicator.
- Acids do not affect turmeric (it stays yellow).
- Bases (like soap) turn turmeric red.
Q7: What happens when we dip litmus paper in different solutions?
| Test Solution | Effect on Red Litmus Paper | Effect on Blue Litmus Paper | Inference (Acidic/Basic/Neutral) |
| Lemon juice | No change | Turns red | Acidic |
| Vinegar | No change | Turns red | Acidic |
| Baking soda | Turns blue | No change | Basic |
| Soap solution | Turns blue | No change | Basic |
| Sugar solution | No change | No change | Neutral |
| Common salt solution | No change | No change | Neutral |
Q8: What happens when China Rose Indicator is added to acids and bases?
- In acids: The China Rose indicator turns dark pink (magenta).
- In bases: The China Rose indicator turns green.
Q9: What is neutralization?
- Neutralization is the reaction between an acid and a base to form salt and water.
- Example: Hydrochloric acid (HCl)+Sodium hydroxide (NaOH)→Sodium chloride (NaCl)+Water (H₂O)\text{Hydrochloric acid (HCl)} + \text{Sodium hydroxide (NaOH)} \rightarrow \text{Sodium chloride (NaCl)} + \text{Water (H₂O)}
- Heat is also produced in this reaction.
Q10: Give some daily life examples of neutralization.
| Situation | Explanation |
| Indigestion | Too much stomach acid causes indigestion. Milk of Magnesia (Mg(OH)₂) neutralizes the acid. |
| Ant Bite | Ants inject formic acid. Baking soda (NaHCO₃) or calamine lotion (ZnCO₃) neutralizes it. |
| Soil Treatment | Acidic soil is treated with lime (CaO) or slaked lime (Ca(OH)₂). Basic soil is treated with organic compost. |
| Factory Waste | Acidic factory waste is neutralized before disposal to protect water bodies. |
Exercise Questions & Answers – Acids, Bases, and Salts
1. State the differences between acids and bases.
| Property | Acids | Bases |
| Definition | Substances that produce hydrogen ions (H⁺) in water. | Substances that produce hydroxide ions (OH⁻) in water. |
| Taste | Sour | Bitter |
| Touch | Not slippery | Soapy and slippery |
| Effect on Litmus Paper | Turns blue litmus red | Turns red litmus blue |
| Reaction with Metals | Produces hydrogen gas | Does not react with metals |
| Reaction with Bases | Neutralizes bases to form salt and water | Neutralizes acids to form salt and water |
| Examples | Lemon juice, vinegar, curd | Soap, baking soda, lime water |
2. Ammonia is found in many household products, such as window cleaners. It turns red litmus blue. What is its nature?
- Ammonia is a base because it turns red litmus paper blue.
- It is commonly used in window cleaners due to its cleaning properties.
3. Name the source from which litmus solution is obtained. What is the use of this solution?
- Litmus solution is obtained from lichens (a type of plant).
- Use: It is used as an indicator to determine whether a substance is acidic or basic.
- Acids turn blue litmus red.
- Bases turn red litmus blue.
4. Is distilled water acidic, basic, or neutral? How would you verify it?
- Distilled water is neutral because it does not contain acids or bases.
- Verification:
- Take red and blue litmus paper.
- Dip them in distilled water.
- No color change means the water is neutral.
5. Describe the process of neutralization with the help of an example.
- Neutralization is the reaction between an acid and a base to form salt and water.
- This reaction removes the acidic and basic properties of the substances.
- Heat is also produced in neutralization, making it an exothermic reaction.
Hydrochloric acid (HCl)+Sodium hydroxide (NaOH)→Sodium chloride (NaCl)+Water (H₂O)\text{Hydrochloric acid (HCl)} + \text{Sodium hydroxide (NaOH)} \rightarrow \text{Sodium chloride (NaCl)} + \text{Water (H₂O)}
- In this reaction:
- HCl (acid) reacts with NaOH (base).
- Forms salt (NaCl) and water (H₂O).
- Heat is released.
6. Mark ‘T’ if the statement is true and ‘F’ if it is false:
| Statement | True/False | Correction (if false) |
| (i) Nitric acid turns red litmus blue. | False | It turns blue litmus red. |
| (ii) Sodium hydroxide turns blue litmus red. | False | It turns red litmus blue. |
| (iii) Sodium hydroxide and hydrochloric acid neutralize each other and form salt and water. | True | – |
| (iv) An indicator shows different colors in acidic and basic solutions. | True | – |
| (v) Tooth decay is caused by the presence of a base. | False | Tooth decay is caused by acids from bacteria in the mouth. |
7. Dorji has a few bottles of soft drinks in his restaurant. Unfortunately, they are not labeled. One customer wants an acidic drink, another wants a basic drink, and a third one wants a neutral drink. How will Dorji decide which drink to serve to whom?
Dorji can use litmus paper to test the drinks:
- If the drink turns blue litmus red → Acidic drink
- If the drink turns red litmus blue → Basic drink
- If there is no color change → Neutral drink
8. Explain why:
(a) An antacid tablet is taken when you suffer from acidity.
- Stomach produces hydrochloric acid (HCl) for digestion.
- Excess acid causes acidity and discomfort.
- Antacids (e.g., milk of magnesia – Mg(OH)₂) neutralize the excess acid and relieve indigestion.
(b) Calamine solution is applied on the skin when an ant bites.
- Ants inject formic acid when they bite.
- Calamine (zinc carbonate – ZnCO₃) neutralizes the acid, reducing pain and irritation.
(c) Factory waste is neutralized before disposing of it into water bodies.
- Factory waste contains acids, which can harm aquatic life.
- Neutralizing it with a base makes it safe for disposal.
9. Three liquids are given: hydrochloric acid, sodium hydroxide, and sugar solution. How will you identify them using only turmeric indicator?
- Turmeric indicator turns red in a base but stays yellow in acids and neutral substances.
| Liquid | Effect on Turmeric Indicator | Inference |
| Hydrochloric acid (HCl) | No change (stays yellow) | Acidic |
| Sodium hydroxide (NaOH) | Turns red | Basic |
| Sugar solution | No change (stays yellow) | Neutral |
Conclusion:
- The liquid that turns turmeric red is sodium hydroxide (NaOH) (base).
- The liquids that do not change color are hydrochloric acid (HCl) and sugar solution.
- To further confirm, we can test HCl with blue litmus (it turns red).
10. Blue litmus paper is dipped in a solution. It remains blue. What is the nature of the solution? Explain.
- If blue litmus remains blue, the solution is either basic or neutral.
- Acids turn blue litmus red, but bases and neutral solutions do not affect it.
- To confirm:
- Test with red litmus paper.
- If red litmus turns blue → The solution is a base.
- If red litmus remains red → The solution is neutral.
11. Consider the following statements:
| Statement | Correct or Incorrect? |
| (a) Both acids and bases change the color of all indicators. | Incorrect |
| (b) If an indicator gives a color change with an acid, it does not give a change with a base. | Incorrect |
| (c) If an indicator changes color with a base, it does not change color with an acid. | Incorrect |
| (d) Change of color in an acid and a base depends on the type of indicator. | Correct |
Correct Option: (iv) Only d is correct.
Useful links: